What Is the Approximate Mass of One Proton
Each electron has a negative charge -1 equal to the positive charge of a proton 1. 11 u 3 l g.

The Structure Of The Atom Boundless Chemistry
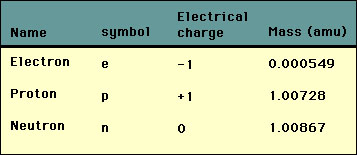
Assume 1 proton weighs 1 amu 1 neutron weighs 1 amu and 1 electron weighs 548 x 104 amu.

. What is the approximate mass of one proton. A48 g piece of ice at 00 c is added to a sample of water at 74 c. Protons and neutrons have nearly the same mass while electrons are much less massive.
This is a tiny dense region at the center of the atom. AThe atomic number is 9 and the mass number is 19. Said another way protons are only about 9986 as massive as neutrons while electrons are only about 0054 as massive as.
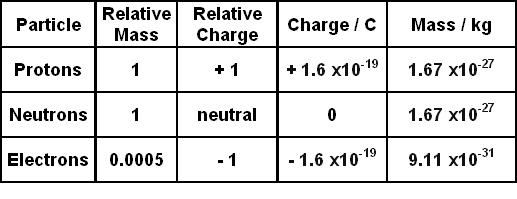
Charge on a proton 1602 10-19 coulombs. Neutrons are uncharged particles found within the nucleus. Charge of 1 and mass of 1 amu.
What is the approximate mass of one proton 100783amu 5 What is the approximate from PSYCHOLOGY NA at Declo Senior High School. In quantum chromodynamics the modern theory of the nuclear force most of the mass of protons and neutrons is explained by special relativity. The mass of a proton is 1840 times the mass of an electron.
What happens if there are too many neutrons in a nucleus. A proton mass is estimated to be 16726 x 10-24 g and the mass of an electron is estimated to be 91093 x 10-28 g. If an atom contains 2 protons 2 neutrons and 2 electrons what is its mass in atomic mass units amu.
Find the momentum and total energy for each particle. Neutron 1 Proton 099862349 Electron 000054386734. B For what values of the momentum of the electron is the total energy within a few percent or of the kinetic energy.
The mass of a neutron is about 1008 664 916 amu. Chemistry 21062019 1330 kellywelly82. How many grams of water were in the sample.
DThe mass of a proton is greater than the mass of a neutron. 4Which statement compares the masses of two subatomic particles. The mass of neutrons and protons are equal to a first approximation and this mass is equal to 1 amu.
What is the approximate mass of. CThe mass of a proton is greater than the mass of an electron. A Consider a photon an electron approximate rest mass 05 MeVc and a proton approximate rest mass1000 MeVc when they each have total kinetic energy 10 eV.
Thus it has a unit positive charge. A proton is one of three main particles that make up the atom. The mass of a proton is 16726231 x 10²⁷ kg whereas the mass of the neutron mn 16726231 x 10²⁷ kg Mass of Neutron in Grams We know the mass of neutron in kg is 16726231 x 10²⁷ Kg.
Proton-has 1 charge -in center -positive proton Related questions. Other questions on the subject. The extra energy of the quarks and gluons in a proton as compared to the rest energy of the quarks alone in the QCD vacuum accounts for almost 99 of the protons mass.
2 00005 u 4 00005 g. What is the charge and mass of a proton. What subatomic particle has an approximate mass of 11840.
Experimental evidence indicates that the nucleus of. 5 rows Mass of the proton is the sum of the mass of current quarks and the binding gluons. Where is the neutron located.
Compared to an electron which particle has a charge. The major portion of an atoms mass nucleus consists of. If we assume that a neutron has a mass of 1 then the relative masses are.
What is mass of proton and neutron. The mass of a proton is about 80100 times greater than the sum of the rest masses of its three valence quarks while the gluons have zero rest mass. Mass of proton 1676 10-27 kg 1676 10-24 g 16726219 10-27 kg.
14 rows 1 What is the approximate mass of a proton. BThe atomic number is 9 and the mass number is 20. Charge of Proton The charge of a proton is equal to and opposite to that of an electron.
Protons have a positive electrical charge of one 1 and a mass of 1 atomic mass unit amu which is about 167 10 27 kilograms. CThe atomic number is 11 and the mass number is 19. Protons are found in the nucleus of the atom.
Protons and neutrons have approximately the same mass about 167 10-24 grams which scientists define as one atomic mass unit amu or one Dalton. The mass of a proton is about 1007 276 466 amu. All of the ice melts and the temperature of the water decreases to 00 c.
Was this answer helpful. How many times larger is the mass of a. The rest mass of a prot.
The mass of a proton 100727amuThe mass neutron 100865amuThe mass of electron 000055amu. Contains most of the mass of the atom. Electron-has a -1 charge-Electrical electron-moves fast AROUND center of atom.
What Is The Relative Mass Of A Neutron Electron And A Proton Quora
How Does The Mass Of An Electron Compare To The Mass Of A Proton Socratic
Comments
Post a Comment